On Dec. 8, FDA approved a long-acting antibody combination that was discovered at Vanderbilt University Medical Center (VUMC) and developed by AstraZeneca.
The combination of two monoclonal antibodies, called Evusheld, was authorized to prevent COVID-19 in adults and children 12 years and older who have compromised immune systems or a history of severe adverse reactions to a COVID-19 vaccine.
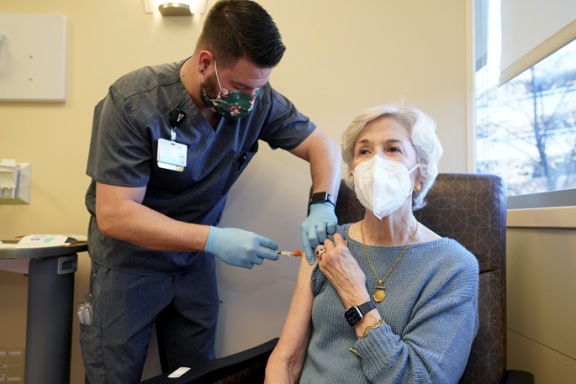
On Dec. 22, Caroline Davis of Nashville became the first patient at VUMC to receive injections of Evusheld to protect her from COVID-19.
James Crowe, MD, Robert Carnahan, PhD, and their colleagues in the Vanderbilt Vaccine Center discovered the antibodies, which were licensed to AstraZeneca in June 2020 for advancement into clinical trials.
Davis, who is being treated for cancer at VUMC, said she could not produce antibodies against the COVID-19 virus on her own, despite receiving two doses of a COVID-19 vaccine and a booster. That's because the chemotherapy she is receiving suppresses her immune system.
“It’s very exciting,” she said, before she received two injections of the antibodies as an outpatient at VUMC. “This is the best Christmas present that could possibly have been given to me."
“We are proud of Vanderbilt’s contributions in the battle against COVID-19," said Jeff Balser, MD, PhD, President and Chief Executive Officer of VUMC and Dean of Vanderbilt University School of Medicine. "The antibodies discovered by Dr. Crowe’s team offer additional protection for millions of immunocompromised people who are not fully protected by the vaccines and have been at risk for severe infection throughout the pandemic."
Learn more about the treatment in this VUMC Reporter article by Bill Snyder.